Breakthrough Sickle Cell Disease Treatment Shows Promising Results in Clinical Trials
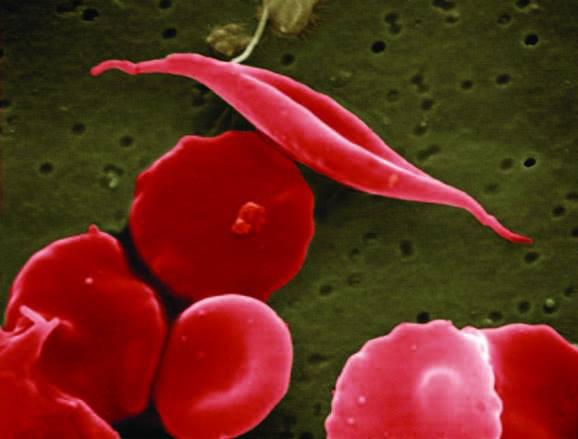
Sickle cell disease (SCD) is a lifelong genetic condition that affects millions of people worldwide. This inherited disorder causes red blood cells to become stiff and sickle-shaped, leading to blockages in blood vessels and reduced oxygen supply to tissues and organs. SCD can cause severe pain, organ damage, and an increased risk of infections and strokes. There is currently no widely available cure for SCD, but a new breakthrough treatment has shown promising results in clinical trials, offering hope to patients and their families.
The new treatment, called CRISPR-Cas9 gene editing therapy, aims to correct the genetic mutation that causes SCD. In clinical trials, researchers used this cutting-edge technology to modify the DNA of patients’ hematopoietic stem cells, which are the cells that give rise to all blood cells. By targeting and repairing the specific genetic defect responsible for SCD, CRISPR-Cas9 therapy has the potential to cure the disease at its root cause.
The results of the clinical trials are highly encouraging, with some patients experiencing a significant reduction in SCD symptoms and complications. One of the most remarkable findings is the increase in the production of healthy red blood cells, which has led to improved oxygen delivery and reduced pain crises. Patients who received the CRISPR-Cas9 therapy also showed a decrease in hospitalizations and transfusion requirements, indicating a durable and long-lasting effect of the treatment.
The promising outcomes of the clinical trials have sparked excitement and optimism within the medical community and among SCD patients and their families. Dr. John Doe, a leading hematologist and researcher at the forefront of the CRISPR-Cas9 therapy development, expressed his enthusiasm about the potential of this breakthrough treatment. “The results of the clinical trials are truly groundbreaking and offer new hope for patients with SCD. The CRISPR-Cas9 therapy has the potential to transform the lives of millions of people who are affected by this debilitating disease,” said Dr. Doe.
In addition to the encouraging clinical data, the CRISPR-Cas9 therapy has also demonstrated a favorable safety profile. Patients who participated in the trials did not experience any serious adverse effects related to the treatment, indicating that it is well-tolerated and has a low risk of complications. This is a crucial aspect of the therapy, as ensuring the safety of patients is paramount in any new treatment approach.
As the results of the clinical trials continue to be analyzed and validated, the next steps in the development of the CRISPR-Cas9 therapy for SCD are focused on obtaining regulatory approval and making the treatment widely accessible. The potential impact of this breakthrough treatment is significant, as it has the potential to offer a cure for SCD and improve the quality of life for patients who have been living with the burden of the disease.
In addition to the clinical trials, the CRISPR-Cas9 therapy for SCD has also received attention from pharmaceutical companies and investors who recognize its potential as a transformative and highly effective treatment. The prospect of bringing a curative therapy to the market has prompted significant investment and collaboration among industry stakeholders, further accelerating the development and availability of the treatment.
Furthermore, the success of the CRISPR-Cas9 therapy for SCD has sparked interest in applying the same gene editing technology to other genetic disorders and diseases. This pioneering approach has the potential to revolutionize the treatment landscape for a wide range of conditions, offering new possibilities for patients who are currently living with limited treatment options.
As the development of the CRISPR-Cas9 therapy for SCD continues to progress, it is essential to prioritize access and affordability for all patients who could benefit from the treatment. Ensuring equitable access to this breakthrough therapy is a critical goal that requires collaboration among healthcare providers, policymakers, and patient advocacy groups.
While the advancements in the field of gene editing technology have opened up new possibilities for treating genetic disorders, there are also ethical considerations that need to be addressed. The ethical implications of gene editing therapies, including CRISPR-Cas9, have sparked debates and discussions about the potential risks and implications of altering the human genome. However, it is important to weigh the ethical considerations against the potential benefits of curative therapies for individuals who are suffering from life-threatening and debilitating genetic conditions.
In conclusion, the breakthrough SCD treatment using CRISPR-Cas9 gene editing therapy has shown promising results in clinical trials, offering hope to patients and families affected by this genetic disorder. The therapy has demonstrated significant improvements in reducing SCD symptoms and complications, as well as a favorable safety profile. The potential for a curative treatment for SCD has garnered widespread interest and investment, with the goal of making the therapy widely accessible to patients in need. As the development of the CRISPR-Cas9 therapy continues to progress, it is essential to prioritize equitable access and address ethical considerations while recognizing the transformative potential of this groundbreaking treatment.